Superplasticizers
Comb-like copolymer polycarboxylates (PC) are known as a new generation of superplasticizers for use in cement suspensions. PCs consist of a backbone and several side chains (brush copolymer). At high pH, the backbone of the polymer chain is charged negatively (due to the deprotonated carboxylate groups), whereas the PEO side chains are neutral. The backbone and side chain lengths of the PC can be determined via gel permeation chromatography with light scattering detection. A schematic illustration of the structure of PC is given in Fig. 1.

Fig. 1: Schematic drawing of the structure of a PC.
A more precise solution structure of PC can be determined utilizing the model according to GAY. The solution structure of PC depends on the side chain density (N = number of monomers per segment), side chain length (nEO = number of ethylene oxide units in the side chain) and the average backbone length (n). By means of these parameters worm- (FBW, SBW) or star-like PCs (FBS, SBS) may be classified (Fig. 2).
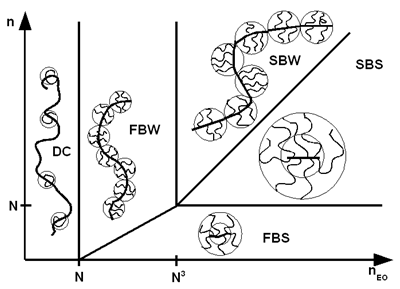
Fig. 2: Solution structures of polycarboxylates (model according to GAY).
Adsorption
PCs can adsorb on charged particle surfaces due to their negatively charged main chain. Besides the adsorption on inert inorganic rheological additives, e.g. limestone powder, particularly the adsorption behaviour of PC on cement is of interest. Both the amount of adsorbed polymer and the adsorption conformation (tail, loop, train, Fig. 3) affect the previously described solution structure of PC. Thereby the side chain length, the side chain density and the specific anionic charge density (charge titration experiment) of the PC in the cement slurry pore solution play an important role. While the adsorbed amount of polymer is readily available via TOC, conclusions concerning the adsorption conformation of the PC can be drawn from zeta potential measurements. Both the amount and the conformation of adsorbed polymer determine the thickness and density of the polymer layer formed. This layer in turn defines the dispersing ability of the suspension, because of the dispersing ability of the PC being due to sterical hindrance between the PEO side chains adsorbed on adjacent cement grains.
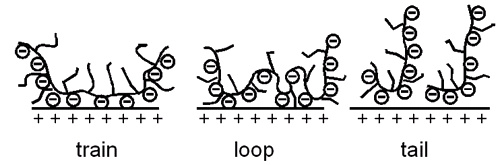
Fig. 3: Possible adsorption conformations of a polymer on an inorganic positively charged surface.
Absorption (Intercalation)
Another interesting interaction of superplasticizers with hydrate phases in cementitious systems is, besides adsorption on the hydrating cement grain, the absorption, namely intercalation. The PC as an anionic compound can be intercalated into the positively charged tricalciumaluminate (C3A) – hydrate layers resulting in charge neutralisation. As previously described, the adsorption of the PCs on the grain surface is responsible for the dispersion of the cement grains. In the case of absorption, the PCs are intercalated into the hydrate phases and are no longer available for the dispersing effect. The resulting composite materials are characterized by a significant expansion of the layers of the lamellar structure of the C3A hydrate phase (Fig. 4). Via different analytical methods (PXRD, IR, TG, elemental analysis) these intercalates can be examined and characterized.
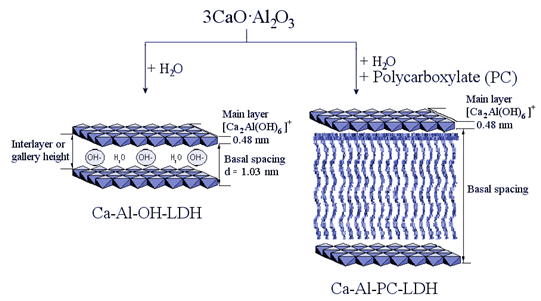
Fig. 4: Schematic illustration of the C3A hydration in presence of water (left) and polycarboxylate (right).
Research focus

