Effect of Microgravity on Early Cement Hydration
 |
Ordinary Portland cement constitutes a mixture of different clinker phases (tricalcium silicate, dicalcium silicate, tricalcium aluminate and calcium aluminateferrite). Addition of water to cement initiates the hydration process. The hydration products present the anchoring sites for concrete admixtures. One of the first products formed within a few seconds of cement hydration is ettringite. The influence of microgravity on the early stage of cement hydration was studied in the presence and absence of chemical admixtures. Cement hydration experiments were performed over 10 s during parabolic flights and for comparison, the experiments were carried out under normal gravity conditions. Four cement samples were tested.
The microgravity experiments revealed that under those conditions, generally smaller ettringite crystals, but in larger quantity are formed. Furthermore, the absence of convection leads to a slower crystal growth resulting from diffusion-limited ion transport. Experiments on ettringite crystallization in the presence of polycarboxylate (PCE) superplasticizers suggest that these polymers preferentially adsorb onto the lateral faces of ettringite crystals.
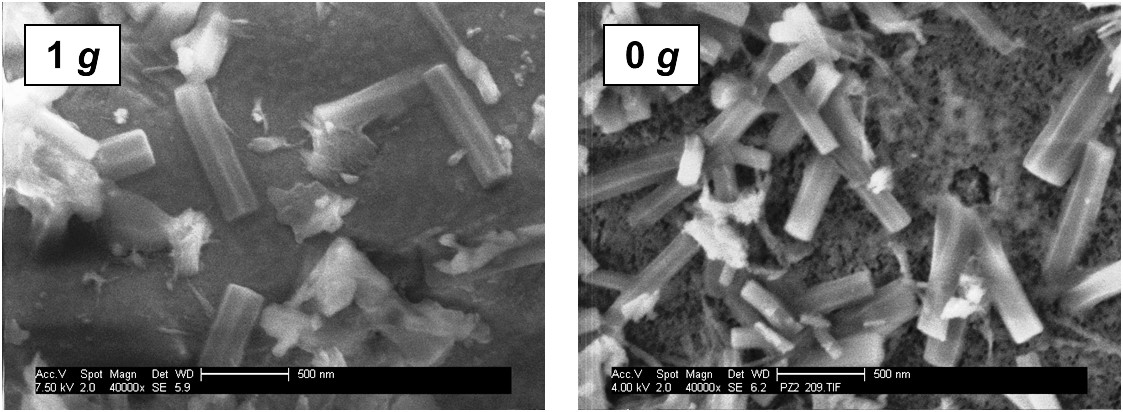
Fig. 1: SEM images of an ordinary Portland cement sample hydrated for 10 s under terrestrial (left) and zero gravity (right) conditions.
In additional experiments, the spontaneous formation of calcium silicate hydrates (C-S-H phases) from combined solutions of Ca(NO3)2 and Na2SiO3 was investigated. C-S-H presents the hydration phase which is mostly responsible for the compressive strength of hardened cement and thus its nucleation and crystal growth is of utmost interest. Under microgravity and at pH = 11.6, nano-sized foils of C-S-H were obtained whereas at pH = 10.5, globular nanoparticles were found. Surprisingly and opposite to this, in the presence of PCE superplasticizers always globular C-S-H nanoparticles were obtained independent of the pH value.
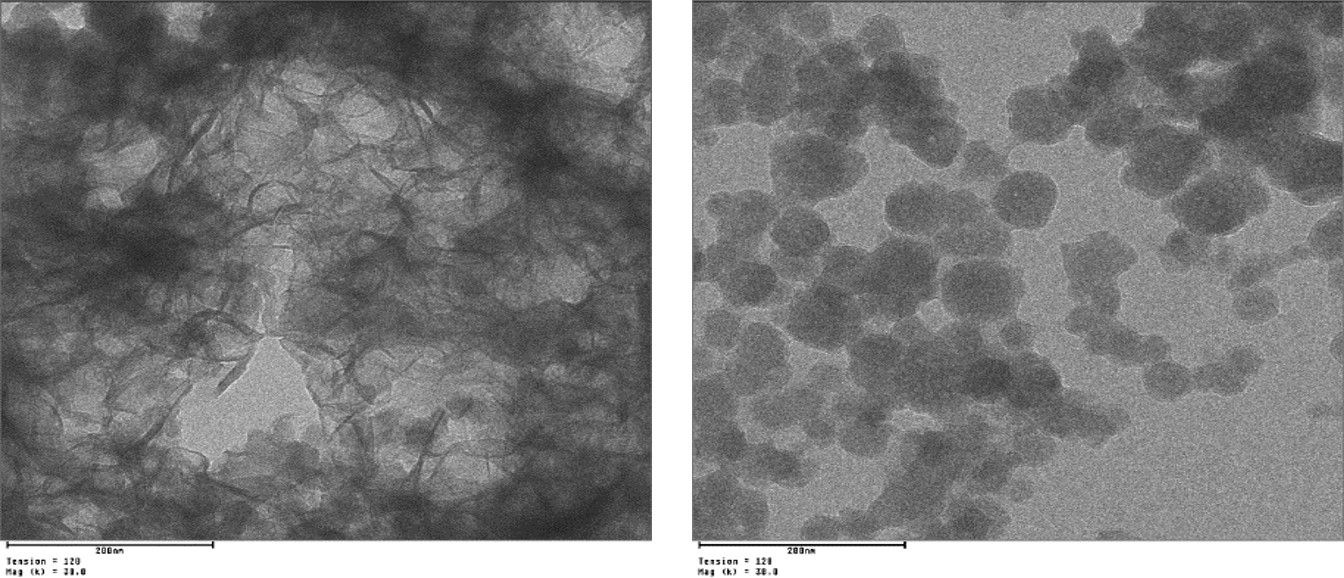
Fig. 2: TEM images of C-S-H phases obtained after 10 sec of crysatallization under zero gravity in the absence (left) and in the presence (right) of a PCE superplasticizer.

